Electric vehicles (EVs) are all the rage. They are touted as a cleaner and more sustainable alternative to gasoline-powered vehicles. The United States government has passed numerous legislative actions to phase out gas-powered vehicles and make sure that within the near term all vehicles are electric.
The common belief is that EVs are more environmentally friendly and in general a safer form of energy. The dark secret: Neither is true.
Currently the gold standard for all major electronic products, EVs included, are batteries made of “lithium ion.” The inventor of lithium ion himself, after receiving the Nobel Prize, warned the world of the dangers of the lithium-ion batteries. So what are they?
ENVIRONMENTAL IMPACT
The main environmental concern associated with lithium-ion batteries is the mining of the raw materials needed to produce them, which can have significant environmental impacts. Lithium is typically extracted from salt flats, which can disrupt local ecosystems and contaminate groundwater. Cobalt and nickel, which are also used in lithium-ion batteries, are typically mined in countries with lax environmental and labor standards, which can lead to environmental damage and human rights abuses.
The production of lithium-ion batteries also requires a significant amount of energy, which is typically generated from nonrenewable sources such as coal and natural gas. This means that the production of EVs can actually result in higher greenhouse gas emissions than the production of gas-powered vehicles.
Coal and natural gas, which are required to generate the power that charges lithium-ion batteries, are supplied through plants that in themselves are releasing dangerous toxic waste—in some cases more than just burning regular gasoline.
Then there is the decomposition period. How long does it take for that used lithium-ion battery to decompose? Five years? Ten? The answer: 100 or more years. Simply put, for 100 years after a battery has been used up, it is slowing releasing potentially toxic by-products into the environment, causing potentially more pollutants and more damage than traditional gasoline.
When these batteries are no longer useful, they can release toxic chemicals into the environment if they are not disposed of properly. This can lead to soil and water pollution, as well as harm to human health.
Another major drawback of lithium-ion batteries is their limited life span. Over time, the performance of the battery deteriorates and, eventually, it needs to be replaced. The life span of a battery depends on a variety of factors, including the number of charge and discharge cycles it undergoes, the temperature at which it operates, and how it is used. For example, exposing a battery to high temperatures or overcharging it can significantly reduce its life span. This leads to a continuous stream of battery waste that needs to be properly disposed of or recycled. The recycling process for lithium-ion batteries is also energy intensive and can release toxic chemicals into the environment if not done properly.
But there is more.

THE DANGERS OF LITHIUM ION BATTERIES
I am going to make a bold statement. But it is one that is true. EVs are basically a bomb on wheels, because of the lithium-ion batteries. There I said it. I am sure it’s not a popular statement, but a factual one nonetheless.
Lithium-ion batteries, as confirmed by their own inventor, are seriously flawed and seriously dangerous.
Lithium-ion battery fires occur due to a number of factors, including manufacturing defects, physical damage, overcharging or overheating, and exposure to high temperatures or fire. A lithium-ion battery is more dangerous than an open gas tank filled with gas. Not only can the smallest penetration cause them to explode, but a minor overheat or even outside temperatures being too high can cause them to catch fire. A lithium-ion battery fire releases large amounts of heat, smoke, and toxic gases, which can pose a serious risk to human health and the environment.
One of the main reasons why lithium-ion fires are so dangerous is the rapid rate at which they can release energy. When a lithium-ion battery ignites, the heat generated can cause the electrolytes to break down, releasing large amounts of oxygen and hydrogen gas. This reaction can cause the battery to rupture or explode, releasing even more heat and toxic gases.
We have all seen race cars with fire extinguishers inside. In fact, many older cars had small fire extinguishers just in case, and many of us have emergency kits in our trunks with a small fire extinguisher. Whether gas or powder, they do not work on lithium-ion fires. Rather, they make them worse. Lithium-ion fires are nearly impossible to extinguish. This is because these materials can cause the battery to release even more flammable gases, which can reignite and cause the fire to spread.
The National Transportation Safety Board has conducted tests on lithium-ion battery fires in EVs and found that these fires can reach temperatures of over 1,000 degrees Celsius (1,832 degrees Fahrenheit) and burn for hours or even days. In some cases, the batteries can reignite even after being extinguished, making them difficult to fully extinguish.
The duration and intensity of a lithium-ion fire depends mostly on the number of batteries in car. So how many batteries are in an average electric car?
Since Teslas are by far the most popular EV, I decided to look at the Tesla models to see how many total batteries are in one car.
The Model S and Model X battery packs contain over 7,000 individual cells (batteries), while the Model 3 battery pack contains around 4,000 cells (batteries).
The smallest of car accidents can trigger just one of the 4,000–7,000 batteries—as can a hot day, an internal leak, a nail that fell onto the highway and finds its way into the undercarriage—and once one battery goes, they all go. A chemical fire of 1,000+ degrees cannot be put out by any traditional method and burns for hours or days.
Furthermore, the toxic gases released by lithium-ion battery fires can pose a serious risk to human health. These gases can include carbon monoxide, carbon dioxide, and hydrogen fluoride, which can cause respiratory problems, skin and eye irritation, and other health effects.
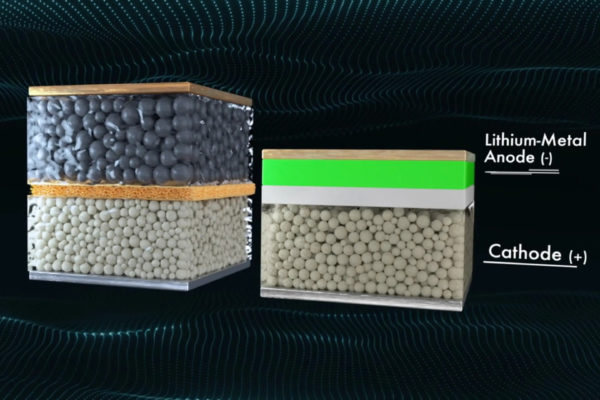
QuantumScape’s solid state battery (right) stores far more energy per weight and volume than regular lithium-ion cells (left), and has just passed a series of EV-related tests with flying colors
IF NOT GAS AND NOT LITHIUM ION, THEN WHAT?
A trillion-dollar race is underway to find a new form of energy to replace lithium-ion. Everything from cold-fusion to the idea of a “perpetual battery” is being worked on. The biggest names in technology are all in a race to solve this problem. Bill Gates is one of them. He recently took public a company called QuantumScape, whose goal is to create a “solid-state battery.” They have raised billions of dollars of capital and currently have a market cap of $20 billion.
What have they accomplished? As of today, QuantumScape’s battery is more theoretical than executable. Its mission statement is to create a solid-state battery that uses a unique architecture and proprietary materials to achieve a number of advantages over conventional lithium-ion batteries. For example, solid-state batteries are expected to have higher energy density, which means they can store more energy in the same amount of space. This could enable longer driving ranges for EVs, as well as longer run times for portable electronics.
In addition, solid-state batteries are expected to be safer than lithium-ion batteries, which can be prone to thermal runaway and catch fire. Solid-state batteries are less likely to catch fire and are less likely to release toxic chemicals or gasses if they do catch fire.
While an exciting prospect, there has been no indication that QuantumScape has a plan to put its technology or batteries into mass production.

Nanotech has perfected a high-performance graphene-powered battery that can withstand the volume changes of the battery electrodes during charge and discharge, greatly reducing the chances of an internal short circuit, which leads to a safe and more powerful battery. Source: PRNewsfoto/Nanotech Energy Inc.
THE HOLY GRAIL? GRAPHENE
Graphene is a two-dimensional material consisting of a single layer of carbon atoms arranged in a hexagonal lattice. It is incredibly strong, lightweight, and conductive, making it an ideal material for a wide range of applications.
Graphene is an excellent conductor of electricity, meaning that it can facilitate faster charging and discharging times. It is also much more durable than graphite, so batteries made with graphene anodes could have longer life spans. Furthermore, graphene can store more energy than graphite, making it possible to create batteries with higher energy densities.
Another advantage of using graphene in batteries is that it is a more environmentally friendly material than the metals and other materials used in current lithium-ion batteries. Graphene is made from carbon, which is a readily available and abundant resource, whereas many of the metals used in lithium-ion batteries are scarce and expensive.
Dozens of companies are working on graphene batteries. The biggest challenge that these companies face is that graphene only works in its purest form, that is, a single layer of carbon. Many companies can make graphene in multiple layers, which is simply a fancy word for graphite. And while it has many uses, once an additional lawyer is added its conductivity and properties are significantly diminished. But one company has figured out how to mass produce graphene and is quickly pulling out front in the pole position: Nanotech Energy.
Nanotech Energy has been making headlines in the tech world for its game-changing work in the realm of battery production. The company’s patented graphene-manufacturing technology has positioned it as the exclusive owner of a key material for the creation of safer, more efficient, and faster-charging batteries. Nanotech Energy holds the very first patent for graphene, which was created by Richard Kaner, PhD, who patented the only known manner to chemically create graphene, which means the only known way to mass produce graphene, the foundation for the launch of Nanotech Energy.
Dr. Kaner is a prominent researcher and inventor in the field of nanotechnology, widely known for his pioneering work in the development of graphene. He is also the holder of the patent for graphene, which has the potential to revolutionize a wide range of industries.
His interest in graphene dates back to the 1970s, when he was studying for his PhD in solid-state physics at the University of Pittsburgh. During this time, he became interested in the properties of carbon-based materials and began to explore the potential uses of graphene. Over the years, he continued to study and refine his techniques for producing graphene, and eventually developed a method for creating high-quality graphene sheets that was both scalable and cost-effective.
Dr. Kaner’s groundbreaking work in the field of graphene has been recognized by his peers, and he has received numerous awards and accolades for his contributions to the field of nanotechnology. In addition to his work on graphene, Dr. Kaner has also conducted research in a wide range of other areas, including superconductivity, photonics, and quantum mechanics.
Perhaps most important is that he patented the process for creating graphene in 2002, prior to the world even acknowledging the existence of graphene. That, along with dozens of other patents, is held by Nanotech Energy.
Nanotech Energy has been rapidly expanding its reach in the battery industry, securing close to $1 billion in contracts for its revolutionary technology from household names. The company recently purchased a 500-acre plot in Nevada and has begun construction on phase one of its new graphene factory and rolling out the first graphene-powered batteries.
Nanotech Energy has already produced five generations of batteries and is currently providing batteries to some of the biggest companies in the world. Nanotech Energy has safety tested its batteries and shown them to be noncombustible, with a high safety rating. They store more energy, charge faster, do not lose their charge ability, and do not have the negative environmental impact of their lithium-ion counterparts.
Nanotech Energy won the prestigious 2022 CES Innovation Award.
The future of energy storage is set to be transformed by Nanotech Energy’s revolutionary graphene-based batteries. With enhanced safety features, faster charging times, and longer-lasting performance, these batteries offer a significant advancement over conventional lithium-ion batteries. As the company moves toward mass production, the possibilities for the future of battery technology are endless. Nanotech Energy recently announced an agreement with powerhouse energy provider Smile Energy. It currently has 300 MW of solar developments in Greece, Bulgaria, and Romania, alongside 700 MWh of energy storage in development across the same region. It will now use Nanotech Energy’s nonflammable, safe battery technology to consider developing battery-energy storage systems for private homes, commercial real estate, and the marine sector, and expand to the Balkans and the Middle East.
The race to fix the lithium-ion debacle is on. It is perhaps one of the most lucrative battles of our generation.
According to Grand View Research, the global battery market size was valued at $77.42 billion in 2020 and is expected to grow at a compound annual growth rate of 10.2% from 2021 to 2028. Other reports suggest that the market size could be even larger, with some estimates projecting it to reach over $100 billion by 2025.
Who gets there first is up in the air, but it sure seems that Nanotech Energy is far ahead of the pack—and with $100 billion a year up for grabs, this is surely going to be front and center sooner rather than later.
Ryan Kavanaugh is the Principal and Founder of Proxima Media and Relativity Media, and the co-founder of Triller.